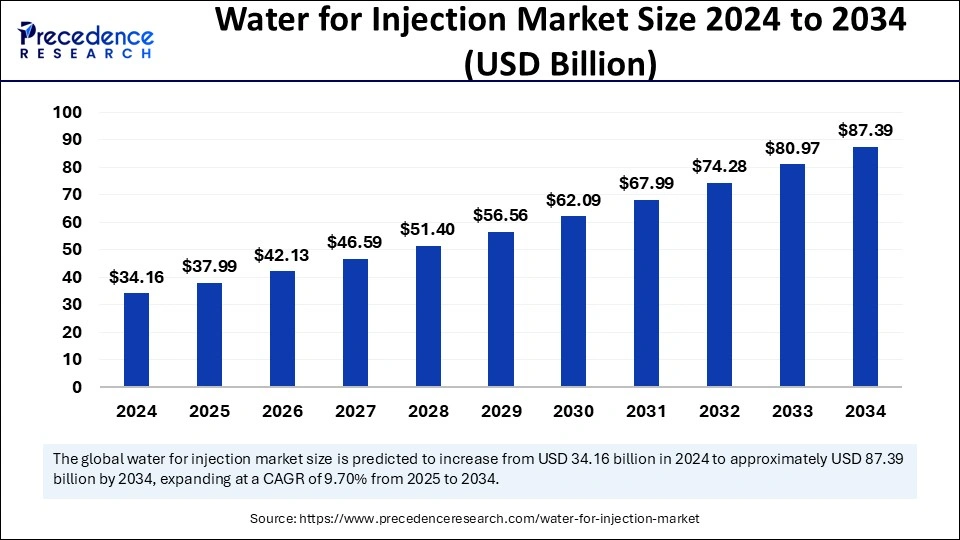
Water for injection market is projected to grow from USD 34.16 billion in 2024 to USD 87.39 billion by 2034, at a CAGR of 9.70%.
Water for Injection Market Key Takeaways
-
North America led the global water for injection market with a 39.72% share in 2024.
-
Asia Pacific is expected to expand at a robust CAGR of 10.07% during the forecast period.
-
The formulated parenteral drugs segment accounted for the highest market share of 65.02% by application in 2024.
-
The solvent segment is projected to grow at the fastest CAGR of 9.76% over the forecast period.
-
Pharmaceutical and biotechnology companies held the largest market share of 43.05% by end-user in 2024.
-
The research institutes segment is forecasted to grow at the highest CAGR of 9.73% during the projected period.
Water for Injection Market Overview
The Water for Injection (WFI) Market is experiencing steady growth due to the increasing demand for sterile and high-quality water in pharmaceutical, biotechnology, and healthcare applications. WFI is a highly purified form of water that meets stringent regulatory standards for use in the preparation of parenteral solutions, intravenous medications, and other pharmaceutical products. It is produced through distillation or reverse osmosis, followed by ultrafiltration and deionization to ensure the removal of microbial contaminants, endotoxins, and other impurities.
The growing emphasis on sterile manufacturing processes, stringent regulatory requirements, and the expanding pharmaceutical industry are driving the demand for WFI globally. The market is poised for continuous growth as pharmaceutical companies, biologics manufacturers, and contract manufacturing organizations (CMOs) increasingly adopt advanced water purification technologies to meet regulatory compliance and ensure product safety.
Water for Injection Market Drivers
-
Growing Demand for Injectable and Parenteral Drugs
The increasing prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders is driving the demand for injectable and parenteral drugs, which require WFI for formulation and manufacturing. The rise in biologics and biosimilars further contributes to the demand for high-quality WFI. -
Stringent Regulatory Requirements for Pharmaceutical Manufacturing
Regulatory authorities such as the FDA, EMA, and WHO mandate strict quality standards for WFI used in pharmaceutical production. Compliance with Good Manufacturing Practices (GMP) and stringent microbiological standards is compelling pharmaceutical companies to invest in reliable WFI production systems. -
Expansion of the Biotechnology and Biopharmaceutical Sectors
The rapid growth of the biotechnology and biopharmaceutical industries, fueled by advancements in gene therapies, cell therapies, and personalized medicine, is increasing the demand for WFI. These industries require ultra-pure water to ensure product safety and efficacy in sensitive therapeutic applications.
Water for Injection Market Opportunities
-
Adoption of Advanced Water Purification Technologies
Technological advancements in water purification, including the adoption of vapor compression, multiple-effect distillation (MED), and membrane-based systems, are creating opportunities for enhancing the efficiency and cost-effectiveness of WFI production. -
Increasing Outsourcing to Contract Manufacturing Organizations (CMOs)
Pharmaceutical companies are increasingly outsourcing WFI production to CMOs to streamline operations and reduce costs. This trend is creating opportunities for CMOs to expand their service portfolios and invest in high-capacity WFI production systems. -
Expansion in Emerging Markets and Developing Regions
Emerging markets in Asia Pacific, Latin America, and the Middle East are witnessing a surge in pharmaceutical manufacturing activities. The growing emphasis on local production of generic drugs and vaccines is driving the demand for reliable WFI systems in these regions.
Water for Injection Market Challenges
-
High Capital Investment and Operational Costs
Setting up and maintaining WFI production systems involves significant capital investment and operational expenses. The high cost of energy, equipment, and compliance with regulatory standards poses a challenge for smaller manufacturers. -
Risk of Microbial Contamination and Endotoxin Presence
Ensuring consistent microbiological purity in WFI systems requires continuous monitoring and maintenance. The risk of microbial contamination and endotoxin presence remains a critical challenge that can impact product safety and regulatory compliance. -
Complex Validation and Qualification Processes
The validation and qualification of WFI systems involve complex procedures that require extensive documentation, testing, and monitoring to ensure compliance with regulatory guidelines. Any deviation from established standards can result in production delays and regulatory penalties.
Water for Injection Market Regional Insights
-
North America
North America dominates the WFI market, driven by the presence of a robust pharmaceutical and biotechnology industry. The region’s stringent regulatory landscape and high demand for injectable and biologic therapies contribute to the strong market growth. -
Europe
Europe holds a significant market share due to the region’s emphasis on maintaining high pharmaceutical manufacturing standards and regulatory compliance. Countries such as Germany, France, and the UK are major contributors to market growth. -
Asia Pacific
Asia Pacific is witnessing rapid growth in the WFI market due to increasing investments in pharmaceutical manufacturing, growing demand for generic drugs, and government initiatives to strengthen healthcare infrastructure. Countries like China, India, and South Korea are driving regional market expansion.
Water for Injection Market Recent Developments
-
Introduction of Membrane-Based WFI Production Systems
Leading manufacturers are introducing membrane-based WFI production systems that offer cost-effective and energy-efficient alternatives to traditional distillation methods. -
Strategic Collaborations for Expanding Production Capacity
Market players are entering strategic collaborations and partnerships to enhance their production capacity and meet the growing demand for WFI in pharmaceutical and biotechnology applications. -
Regulatory Approvals for Novel WFI Production Technologies
Regulatory authorities are approving innovative WFI production technologies that meet stringent quality standards, enabling manufacturers to adopt more efficient and sustainable production processes.
Water for Injection Market Companies
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Eurocrit Labs International
- Evoqua Water Technologies
- Veolia Water Solutions and Technologies
- ICU Medical Inc.
- Danaher Corporation (Cytiva)
- Rocky Mountain Biologicals
- B. Braun Melsungen AG
- SteriCare Solutions
- Veltek Associates, Inc.
Segment Covered in the Report
By Application
- Formulate Parental Drugs
- Solvent
- Cell Culture Media
- Laboratory Reagents
- Synthesis of Drugs
- Others
- Cleaning Agents
- Rinsing Vessels
- Cleaning Equipment
- Cleaned primary Packaging Materials
By End-user
- Pharmaceutical and Biotechnology Companies
- Research Institutes
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!