The global battery free implants market size is estimated to hit around USD 43 55 billion by 2034 increasing from USD 7 99 billion in 2024, with a CAGR of 18.48%.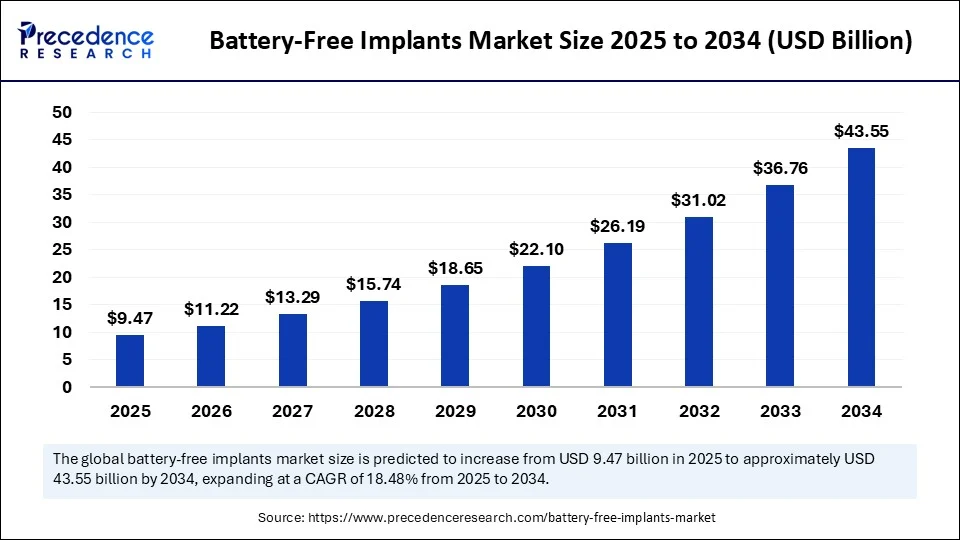
Battery Free Implants Market Key Takeaways
- In terms of revenue, the global battery-free implants market was valued at USD 7.99 billion in 2024.
- It is projected to reach USD 43.55 billion by 2034.
- The market is expected to grow at a CAGR of 18.48% from 2025 to 2034.
- North America dominated the global battery-free implants market in 2024.
- Asia Pacific is expected to grow at the fastest CAGR from 2025 to 2034.
- By application, the cardiac monitoring and pacing devices segment held the major market share in 2024.
- By application, the orthopedic monitoring devices segment is projected to grow at the highest CAGR between 2025 and 2034.
- By therapeutic area, the cardiology segment contributed the biggest market share in 2024.
- By therapeutic area, the orthopedics segment is expanding at a significant CAGR between 2025 and 2034.
- By energy harvesting technology, the radiofrequency (RF)-based devices segment led the market in 2024.
- By energy harvesting technology, the piezoelectric energy conversion segment is expected to grow at a significant CAGR over the projected period.
- By material, composite materials generated the largest market share in 2024.
- By material, bioresorbable materials segment is expected to grow at a notable CAGR from 2025 to 2034.
- By end user, the hospitals and clinics segment held the major market share in 2024.
- By end user, the homecare settings segment is projected to grow at a significant CAGR between 2025 and 2034
Impact of Artificial Intelligence on the Battery-Free Implants Market
Battery-free implants are advancing through intelligent, rapid, and accurate clinical deployment With artificial intelligence at the helm, AI optimizes their energy-harvesting mechanisms—whether tapping into kinetic motion or thermal gradients—to maintain consistent output in the body’s tough environments. By applying AI-based analytics in healthcare, providers can pinpoint patients who will benefit most from these implants. Ultimately, AI is driving the evolution toward greener, less invasive, and patient-specific devices, maximizing function with zero battery dependence
Get Sample Link@ https://www.precedenceresearch.com/sample/6346
Market Overview
Battery‑free implants represent a next‑generation class of implantable medical devices powered entirely through energy harvesting instead of internal batteries. These devices utilize ambient sources—body heat, mechanical motion, blood biochemistry or external RF/ultrasound—to operate continuously. Typical applications include neuromodulation, cardiac pacing, remote diagnostics, drug delivery, and physiological sensing.
Without finite internal power sources, these implants enable ultra-compact form factors, extended lifetimes, minimal patient intervention, and reduced surgical risk. The market spans patient populations requiring lifelong monitoring, such as those with neurological disorders, cardiac arrhythmias, diabetes or musculoskeletal conditions.
Drivers
-
Rising chronic health burden and elderly populations: With more people living longer and managing chronic illnesses, healthcare systems demand long-term, maintenance-free solutions for continuous monitoring and intervention.
-
Preference for minimally invasive interventions: Healthcare strategy is shifting toward procedures that minimize hospitalization and follow-up surgeries, making battery‑free implants ideal for decentralized, outpatient care.
-
Technological breakthroughs in nano‑fabrication and energy conversion: New piezoelectric, triboelectric, thermoelectric and biofuel cell configurations—in combination with micro wireless power —have enabled significant gains in power efficiency and device miniaturization.
-
Integration into telehealth and digital health ecosystems: As remote patient care becomes mainstream, implants that wirelessly transmit data and respond adaptively using AI provide a high value proposition.
-
Favorable regulatory and funding climate: Many health agencies and research funders support medtech innovations that minimize invasive surgery and extend device lifespans.
Opportunities
-
Use in real‑time closed‑loop therapies: Artificial intelligence can analyze sensor data from the implant and automatically adjust therapy—such as stimulator output—tailored to individual patient rhythms and conditions.
-
Strategic alliances with tech companies: Venture collaboration between medtech manufacturers and semiconductor giants or energy‑harvesting component firms offers access to engineering expertise and accelerated scalability.
-
Diversification into broader clinical domains: Applications beyond cardiology or neurology—such as implantable drug delivery, fertility tracking, orthopedic load sensing, and remote digestive system monitoring—are emerging.
-
Biodegradable implant technology: Materials that dissolve safely after implant function completion remove the need for removal procedures and appeal to pediatric or transient diagnostic use cases.
-
Emerging market penetration: Battery-free implants could be especially transformative in markets with limited surgical infrastructure but growing demand for chronic disease management technologies.
Challenges
-
Unpredictable power availability: Body energy harvesting can vary based on physiological fluctuations or patient activity, which may limit device reliability in critical functions.
-
Limited output for high‑power implants: Deep tissue implants or those needing muscular stimulation often exceed the capacity of current harvesters, making full battery independence difficult.
-
Cost and manufacturing complexity: Advanced nanofabrication and highly precise, biocompatible electronic components remain expensive to produce, slowing mass adoption.
-
Scarce long-term data: Rigorous long-term clinical studies extending over many years are still rare. This collision of novelty and limited historical evidence slows clinician trust and payer coverage.
-
Regulatory fragmentation and wireless safety: Varying global requirements on electromagnetic safety, energy emission, and interoperability add complexity to international product development.
-
Security risks from wireless connectivity: Data transmission from inside the body must be safeguarded against external tampering or hacking.
Recent Developments
-
A collaboration between a startup and an additive‑electronics firm resulted in a proof‑of‑concept battery‑free pacemaker small enough (~4 mm) for catheter delivery, operating continuously on nano‑watt power and eliminating leads or internal batteries.
-
Additive manufacturing methods have enabled micro‑scale energy‑harvesting coil circuits achieving high power densities, ideal for optogenetic probes and neural sensors.
-
Researchers developed mechanical metamaterial implants that function without electronics, transmitting wireless force‑sensing signals from a knee implant structure alone.
-
MXene‑based cardiac patches combine low‑impedance bioelectronic interface, wireless energy scavenging and closed‑loop pacing or recording.
-
Passive ultrasonic links have been demonstrated that allow deep‑tissue implants to communicate without electronics, using piezo crystals as ultrasonic transducers.
-
Regulatory frameworks in the U.S., Europe and Asia are increasingly recognizing standards for battery‑free devices, including wireless safety and data integration protocols.
Battery-Free Implants Market Companies
- Abbott Laboratories
- Biotronik SE & Co. KG
- Cochlear Limited
- EBR Systems, Inc.
- Medtronic plc
- NeuroPace Inc.
- Pixium Vision
- Profusa Inc.
- Second Sight Medical Products Inc.
- Stimwave Technologies Inc.
Latest Announcement by Industry Leader
- In April 2025, CELTRO, established in late 2019, is a forward-thinking startup formed by heart rhythm specialists and experts from the semiconductor industry, including seasoned engineers and executives. The company strives to transform the integration of implants within the human body by designing electronic devices that function independently of external power sources. CELTRO’s inaugural initiative involves the development of a 3D-printed, battery-free pacemaker implant utilizing advanced additively manufactured electronics (AME) technology. “We are extremely excited by the progress we’ve made in the development and testing of our battery-free cardiac implant that has been enhanced using Nano Dimension’s AME technology. We are optimistic about the prospects of creating a platform of next-generation in-body electronics that will help shape the future of the medical implant industry” – Dr.-Ing Gerd Teepe, CELTRO Co-Founder & CEO.
Segments covered in the report
By Application
- Neural Stimulation Devices
- Cardiac Monitoring & Pacing Devices
- Drug Delivery Systems
- Bio-sensing and Diagnostics
- Hearing Implants
- Orthopedic Monitoring Device
By Therapeutic Area
- Cardiology
- Neurology
- Orthopedics
- Endocrinology (e.g., Glucose Monitoring)
- ENT (Ear, Nose, Throat)
- Urology & Gastroenterology
By Energy Harvesting Technology
- Radiofrequency (RF)-Based Devices
- Ultrasound Energy Harvesting
- Piezoelectric Energy Conversion
- Magnetic Resonance Coupling
- Thermoelectric & Bioelectric Harvesting
By Material Type
- Biocompatible Polymers
- Titanium & Other Metals
- Ceramic-Based Materials
- Bioresorbable Materials
- Composite Materials
By End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Research & Academic Institutes
- Homecare Settings
- Specialty Clinics
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
Also Visit@https://www.precedenceresearch.com/